(For more information, please refer to Extension publication Preventing Off-target Herbicide Problems in Cotton Fields, W291-A)
Off-target movement of agricultural chemicals, including pasture and right-of-way herbicides, can be detrimental to cotton production. While these herbicides are valuable tools for weed management, off-target damage to cotton often results in expensive fines and/or lawsuits, delayed harvests, reduced yields, and bad publicity for the industry. Fortunately, preventive steps can be taken to avoid these problems.
- Herbicide Selection — Although highly effective on several broadleaf weeds in pastures and rights-of-way, the auxin or growth regulator herbicides can damage sensitive crops if not used properly. The characteristics of these herbicides determine which product to use in different situations.
- Volatile herbicides — change from a liquid to gas or vapor and move away from the target. Typically, dicamba and 2,4-D are more volatile than aminopyralid or picloram.
- Persistence of herbicides — The persistence of herbicides can affect future plans for a field. While dicamba and 2,4-D are highly active on cotton even in minute doses, these materials are relatively non-persistent in soil and in treated pasture grasses and hay. This is not the case with aminopyralid or picloram. Both of these herbicides can stay active in soil, pasture grass and hay for a year or longer
- Water Solubility — Picloram is more soluble than aminopyralid and therefore more likely to be moved off-site by runoff.
- Prevention of Drift — Several factors can contribute to herbicide drift to sensitive areas. physical and vapor, can occur. (Refer to the section “Spray Today?” in this website)
- Physical Drift — can occur when the herbicide is blown away from the target area on windy days and/or in runoff after a hard rain. It is important to monitor weather conditions several days prior to and after spraying to prevent physical drift.
- Vapor Drift — is the movement of spray vapor away from the target after the herbicide has been deposited on the target. It is mainly influenced by air temperature, but also by relative humidity (RH) and herbicide formulation.
- Field Selection — The location, characteristics and history of a field influence future management strategies. A proper risk assessment should be performed before spraying a pas- ture with some of these herbicides. Rains can wash certain herbicides downhill to sensitive areas.
- Sprayer Contamination — A common way for pasture herbicides to make it into cotton fields is through sprayer contamination. Pasture herbicidesare notoriously difficult to rinse from sprayers. Rinsing withwater does not remove all herbicide residues from a sprayer and hoses
- Monitoring — Producers are encouraged to assess the performance of herbicides in pastures and hay fields. Tracking results will guide future decisions for weed control. It is important to keep a log of all applications with dates, products, field locations and weather conditions.
(For more information, please refer to Extension publication Diagnosing Suspected Off-target Herbicide Damage in Cotton, W291-B)
Following proper stewardship recommendations can reduce the impact of off-target herbicides in cotton (see UT Extension fact sheet W 291-A Preventing Off-target Herbicide Problems in Cotton Fields). However, these unfortunate events sometimes occur and diagnosing problems in the field is difficult. Many pasture herbicides mimic the plant hormone auxin, and symptoms can be quite similar.
Images and descriptions on this webpage are intended to highlight characteristic symptomology of each of these broadleaf herbicides on cotton.
The following are descriptions of commonly observed symptoms resulting from exposureto synthetic auxin herbicides:
- Curling — Folding of edge of leaf margins.
- Epinasty — Twisting, bending and/or elongation of stems and leaf petioles.
- Blistering — Appearance of raised surfaces on leaf tissue.
- Chlorosis — Yellowing or whitening of leaves resulting from loss of chlorophyll.
- Necrosis — Browning of tissue resulting from cell death.
Common Name – Chemical Family – Trade Names:
- aminocyclopyrachlor* – Pyrimidine-carboxylic acid – Not registered for use in pastures and hay fields
- aminopyralid – Pyridine-carboxylic acid – Milestone, ForeFront R&P, ForeFront HL, GrazonNext
- picloram – Pyridine-carboxylic acid – Tordon, Surmount, GrazonP+D
- 2,4-D** – Phenoxyacetic acid – Various names and mixtures
- Dicamba – Benzoic acid – Banvel, Clarity, Oracle, Rifle, Brash, Rangestar, Weedmaster
*Products containing aminocyclopyrachlor (MAT28) are registered for non-cropland use, but are not yet registered for use in pastures.
**Picloram, aminopyralid, and dicamba are often sprayed in combination with 2,4-D.
Plants exposed to picloram typically exhibit symptoms relatively soon.
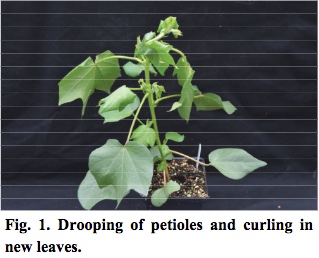
- After three days, leaf petrioles begin to droop. The upper stem is epinastic and newer leaves are folded downwards, nearly vertical. New leaf margins are curled downwards as well (Figure 1).
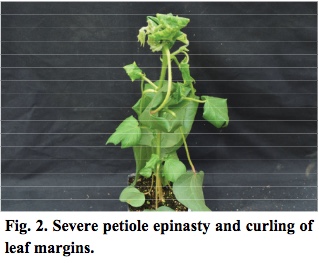
- As early as five days after exposure, new leaves are blistered in appearance. One week after treatment, most petioles are bent downwards, nearly vertical (Figure 2).
- Approximately 10 days after exposure, leaves show signs of chlorosis (Figure 3), and the newest buds are browning (Figure 4).
- At higher rates of exposure, the stem is swollen and ruptured around two weeks after exposure (Figure 5).
- Approximately one month after exposure, nearly half the plant is necrotic (Figure 6).
The following plant images were photographed over time to illustrate the development of symptoms after plants were exposed to picloram.




- Approximately three days after exposure, most petioles are drooping at or below horizontal. Leaves also are curled slightly upwards (Figure 7).
- Within one week, leaf petioles have an increased downward droop. New leaves are yellow and have reduced lateral expansion (Figure 8).
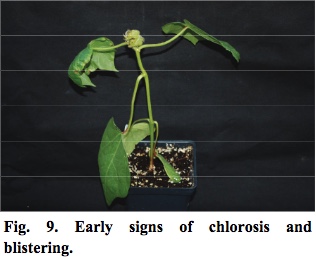
- Within approximately 10 days, plants exposed to high rates of aminocyclopyrachlor show signs of chlorosis and blistering near leaf margins (Figure 9).
- As time goes by, blistering and chlorosis become more apparent and young leaves are cupped upwards (Figure 10). Plants have severely reduced apical growth (Figure 11).
- Six weeks after exposure, younger leaves are brown and older leaves are highly chlorotic (Figure 12). (Abortion of the apical meristem and development of necrotic symptoms are slower than with picloram)
The following plant images were photographed over time to illustrate the development of symptoms after plants were exposed to aminocyclopyrachlor.





Symptoms from exposure to aminopyralid are similar to aminocyclopyrachlor. However, symptoms do not develop as rapidly as with picloram.
- Initially, petioles droop to horizontal approximately three days after treatment (Figure 13).
- Approximately one week after exposure, the main stem is bent and blisters appear near leaf margins (Figure 14).
- After the first week of exposure, petiole epinasty is more pronounced (Figure 15), and new leaves are yellow, blistered and have reduced lateral expansion (Figure 16).
- At low rates of exposure to aminopyralid, older leaves are still upright, but younger leaves are yellow and blistered (Figure 17).
- Six weeks after exposure, older leaves are yellow, younger leaves are browning near the margins, and the apical meristem is aborted (Figure 18).
The following plant images were photographed over time to illustrate the development of symptoms after plants were exposed to aminopyralid.






Injury symptoms of cotton plants exposed to 2, 4-D appear much sooner than picloram, aminocyclopyrachlor or aminopyralid.
- Two days after exposure, leaf petioles are horizontal and the upper stem is bent (Figure 19).
- Within four days, petioles are twisting and new leaves are curled downwards at the margins (Figure 20).
- Days later, red to dark brown patches begin to appear on the stem and petioles (Figure 21).
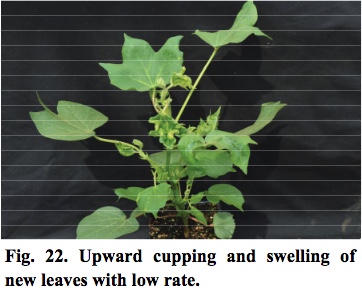
- Lower rates of exposure to 2, 4-D will cause new leaves to cup upwards and blister within two weeks after exposure (Figure 22).
- At one month after exposure, new leaves have parallel venation and lobes have been reduced to finger-like projections (Figure 23). (These young leaves are bent where the base of the leaf meets the petiole and resemble a piece of worn leather.)
- Chlorosis, strapping and reddening of petioles are more severe at six weeks after exposure (Figure 24).
The following plant images were photographed over time to illustrate the development of symptoms after plants were exposed to 2,4-D.





Overall, symptoms develop quickly in plants exposed to dicamba. Genearally, petiole twisting is more severe in plants treated with dicamba than with 2,4-D.
- As soon as two days after exposure, leaf petioles are curved and youngest leaves are curled (Figure 25).
- Approximately one week after treatment, newer leaves are beginning to yellow and blister along the leaf veins. (Figure 26).
- Within 10 days after exposure, petioles are curved severely and often resemble an “S” shape (Figure 27).
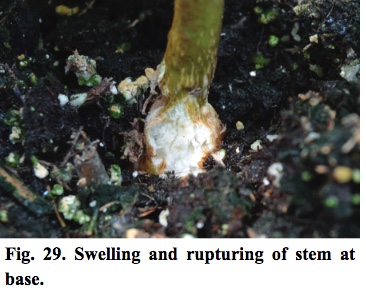
- Later, the newest buds become brown (Figure 28), and the base of the stem has ruptured (Figure 29).
- One month after exposure, older leaves are highly chlorotic and the meristem has been aborted (Figure 30).
The following plant images were photographed over time to illustrate the development of symptoms after plants were exposed to dicamba.





Although diagnosing herbicide injury in the field is difficult, the following steps can be taken to determine possible causes:
- Always record the date, time, location and description of observed symptoms.
- Photographs of injury can help document symptom development, especially since the appearance of plants can change over a short period of time.
- Try to rule out other causes of plant stress, such as weather, soils, insects or misapplied fertilizer.
- Off-target movement of herbicides will cause multiple plants over a large area to exhibit similar symptoms.
- Pay particular attention to leaf margins, new growth, and the main stem, as these areas can offer several clues for herbicide damage.
- If herbicide injury is suspected, it can be difficult to determine if the herbicide was placed there by:
- tank-contamination,
- drift,
- moved well after application due to volatility,
- possibly placed there by manure from livestock who fed on treated forage.
- Research is important to narrow down the source of contamination.
- determine when symptoms first appeared
- whether livestock were given access to the field in the off-season,
- what the previous crop was and what herbicides were applied in the previous three seasons,
- whether manure was used,
- if there was an application of pesticides soon before the symptoms appeared.
- Looking for patterns in fields can also narrow down the source of contamination.
- Scattered patches of herbicide damage may indicate carryover in manure and urine.
- If the majority of plants are injured, then a change in the intensity of symptoms in the field may indicate from which direction the herbicide came.
- Vapor drift can travel several miles, though, making the direction of origin difficult to determine.
- Herbicide residue testing is expensive, especially if the herbicide or family of herbicides is unknown. Being able to narrow the list of possible herbicides can significantly lower the cost of residue testing.
- One important thing to remember is that picloram, aminopyralid and dicamba are often sprayed in combination with 2,4-D. Even though pasture herbicides damage tobacco in similar ways, the descriptions listed on this webpage can help to verify the source of injury
- Rhodes, Jr. G. Neil. Trevor Israel, Larry Steckel. Preventing Off-target Herbicide Problems in Cotton Fields. UT Extension publication W291-A. The University of Tennessee. 2012.
- Rhodes, Jr. G. Neil. Trevor Israel, Larry Steckel. Diagnosing Off-target Herbicide Damage in Cotton Fields. UT Extension publication W291-B. The University of Tennessee. 2012.
- Rhodes, Jr. G. Neil. William Phillips. Weed Control Management in Pasture and Hay Crops. UT Extension publication PB1801. The University of Tennessee. 2012.
- Photo credit: Cotton Field in Tennessee. Digital image. Accessed on 19 May 2018. Available online at http://cdn.shutterstock.com.